High School Science - HS-PS1-7 - Stoichiometry Centered
6 Questions
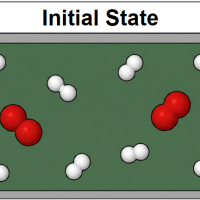
A reaction chamber contains a mixture of hydrogen and oxygen gas as shown in the "Initial State" diagram below.
A student decomposes potassium chlorate to produce oxygen gas and collects the gas over water. Some oxygen may be lost if the collection system leaks.
A lab team produces ammonia using the reaction
A student mixes sodium and chlorine in a sealed container to form table salt. The reaction is:
A student runs a reaction in a sealed container that stays on a balance. The student compares the mass before and after the reaction.
A student mixes 10.0 g CaCO₃ with 15.0 g HCl in a flask connected to a gas syringe. The syringe collects the CO₂ produced. The balanced reaction is:
We help forward-thinking Californian districts achieve significant CAASPP score improvements in Science, Language Arts, and Math. Our NGSS-aligned questions ask students to analyze, model, and reason through real-world science challenges.
Learn more