High School Science - HS-PS1-5 - How Temperature, Concentration, and Catalyst Affect Chemical Reaction Rates
6 Questions
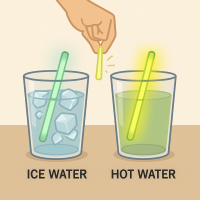
A student snaps two glow sticks of the same brand in two different cups of water.
Students test how a catalyst affects the rate of hydrogen peroxide decomposition (3% H₂O₂ at 25 °C).
Engineers explain that catalytic converters work better once the car engine warms up. Complete the sentences by using the drop-down menus.
A component of urban smog is the brownish gas nitrogen dioxide, which exists in equilibrium with its colorless dimer, dinitrogen tetroxide.
Part A: A student finds a brown plastic bottle of drugstore hydrogen peroxide (3%) in their bathroom cabinet.
Part B: Using your answers from Part A, explain why the hydrogen peroxide solution in the student’s bathroom cabinet became less effective over time.
We help forward-thinking Californian districts achieve significant CAASPP score improvements in Science, Language Arts, and Math. Our NGSS-aligned questions ask students to analyze, model, and reason through real-world science challenges.
Learn more